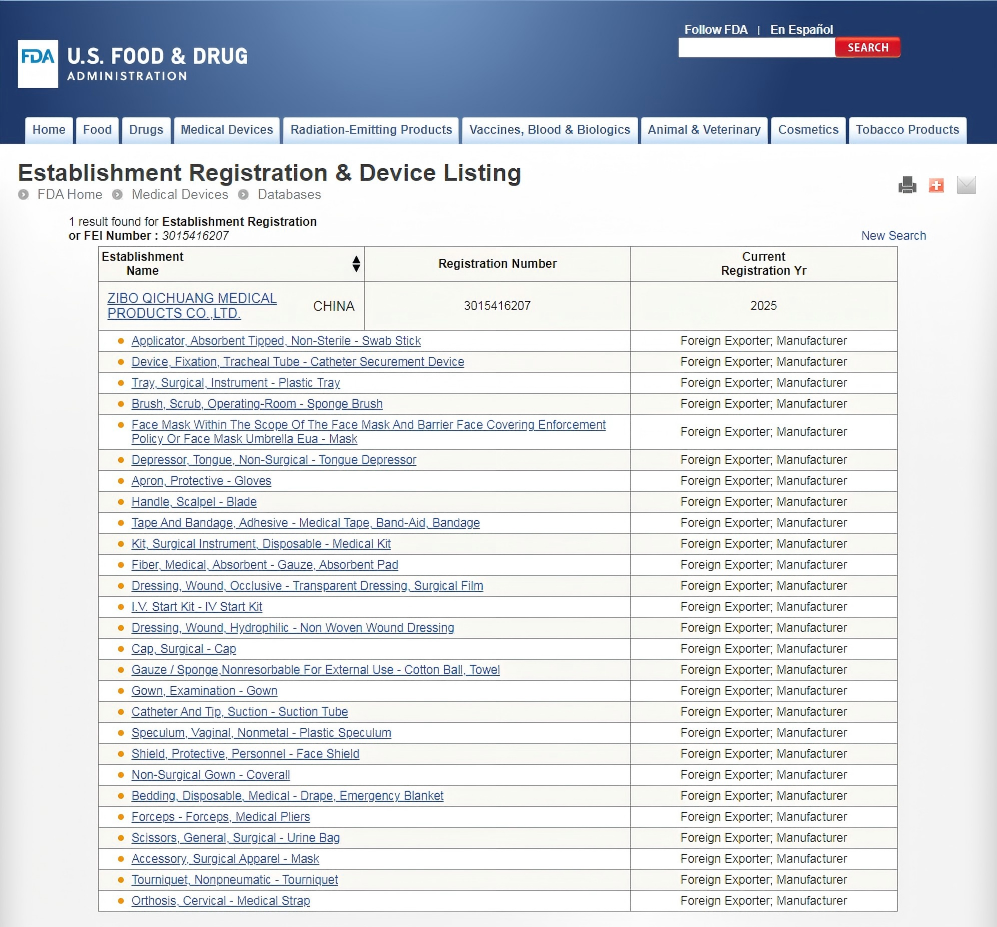
The FDA has requirements for pre-market technical documentation of medical devices, such as risk management, design and manufacturing records etc. During the certification process, the whole company is also constantly learning and pushing the boundaries of innovation.