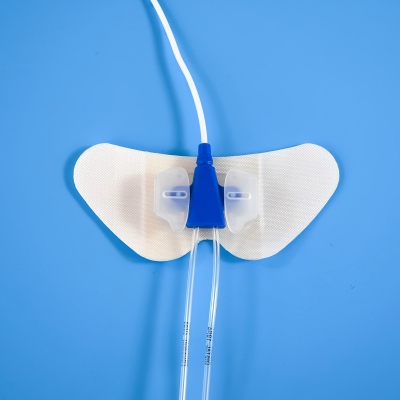
D03091 Securement Device: The Pinnacle of Safety and Simplicity in Vascular Access Management
In the critical care of patients with Peripherally Inserted Central Catheters (PICC) and Central Venous Catheters (CVC), securement is not a mere procedural step—it is a fundamental component of patient safety. Catheter dislodgement or migration can lead to serious complications, treatment interruptions, and increased healthcare costs. The D03091 Sterile Securement Device rises to this challenge with a meticulously engineered design that prioritizes both unwavering security and exceptional patient comfort. Its compliance with ISO, MDR, and FDA standards underscores a universal commitment to the highest levels of quality and safety, making it a globally trusted solution.
A Synthesis of Advanced Features for Optimal Care
The D03091's design is a direct response to the real-world challenges faced by clinicians and patients, transforming the securement process into an experience defined by reliability and ease.
1. Uncompromising Safety and Gentle Adhesion
At the core of its patient-centric design is a hypoallergenic, pressure-sensitive adhesive. This advanced formulation provides a strong, reliable hold while being exceptionally gentle on the skin. It is specifically engineered to minimize the risk of skin irritation, redness, and Medical Adhesive-Related Skin Injuries (MARSI), making it the ideal choice for patients with sensitive skin or those requiring long-term catheterization.
2. Conformable and Water-Resistant for Real-World Resilience
A securement device must perform reliably in dynamic, non-ideal conditions. The D03091 is both conformable and water-resistant. Its flexibility allows it to adhere perfectly to the body's contours, ensuring consistent contact and security even with patient movement. The water-resistant property protects the dressing from compromising due to perspiration or accidental splashes, ensuring integrity is maintained during hygiene activities.
3. Strategic Material Design for Clinical Insight and Discretion
The device's unique translucent and opaque construction offers the best of both worlds. The translucent border allows for continuous visual inspection of the skin and catheter insertion site for early signs of complications, while the opaque securement area provides a clean, discreet appearance that can help reduce patient self-consciousness.
4. Guaranteed Sterility and Intuitive Application
Ethylene Oxide Sterilization: Every device is sterilized using the proven ethylene oxide method, ensuring immediate aseptic readiness for use. This guarantees a sterile field at the critical access site, mitigating infection risk from the moment of application.
Adhesive Side Out When Unrolled: This seemingly simple, yet crucial, design feature streamlines the application process. It allows for easy, one-handed handling, minimizing the risk of contamination from folding or fumbling. This intuitive design promotes aseptic technique, reduces application time, and enhances overall clinical efficiency.
Conclusion: Redefining the Standard of Care
The D03091 Securement Device is more than a piece of tape; it is a sophisticated medical device engineered to uphold the highest standards of vascular access management. By integrating proven safety, resilient performance, and intuitive application, it effectively secures critical lifelines while safeguarding patient skin integrity. It represents a reliable, simple, and intelligent solution that supports clinicians in delivering superior care and provides patients with the comfort and confidence to continue their daily lives.
To learn more about how the D03091 Securement Device can enhance safety and efficiency in your clinical practice, visit เว็บไซต์ : qcmedical.net for comprehensive product information, compliance documentation, and ordering details. For personalized assistance and sample requests, please contact Felix directly.